Determination of Sodium and Barium Content in Strontium Carbonate
1 Sample solution preparation:
2 Experimental equipment and reagents:
Approximately 0.4g sample (precision to 0.0001g) was weighed and placed in a 250mL beaker, 10mL deionized water was added, covered with surface dish. 2mL hydrochloric acid solution was added for digestion, boiled for 2min. After cooling down, it was transferred into a 100mL volumetric flask. 1mL 38% KCl solution, 1mL of hydrochloric acid were added, diluted and made up to the volume with water, shaken well and set aside for later use.
2 Experimental equipment and reagents:
AA7000 series atomic absorption spectrophotometer (with Zn, Al hollow cathode lamp, EWAI Inc.)
Temperature-controlled hot plate
Hydrochloric acid (HCl): excellent grade purity
Potassium chloride (KCl): excellent grade purity
Sodium standard solution (National Reference Materials Research Center)
Barium standard solution (National Reference Materials Research Center)
3 Instrument conditions
4 Standard solution preparation
5 Standard curve
Temperature-controlled hot plate
Hydrochloric acid (HCl): excellent grade purity
Potassium chloride (KCl): excellent grade purity
Sodium standard solution (National Reference Materials Research Center)
Barium standard solution (National Reference Materials Research Center)
3 Instrument conditions
| Parameter | Wavelength (nm) |
Slit width(nm) | Burner height(mm) | Fuel gas flow rate(L/min) | Lamp current(mA) | Flame type |
| Na | 589.0 | 0.2 | 10 | 1.5 | 3.0 | Air – acetylene |
| Ba | 553.6 | 0.2 | 10 | 4.0 | 3.0 | Air – acetylene |
4 Standard solution preparation
| Element | Concentration(μg/mL) | ||||
| Na | 0 | 0.2 | 0.4 | 0.8 | 1.6 |
| Ba | 0 | 5.0 | 10.0 | 15.0 | 25.0 |
5 Standard curve
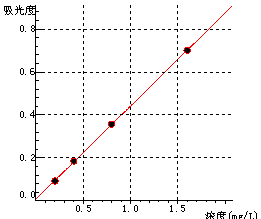 Na Curve equation:y=0.43365*x+0.00834 Linearity coefficient:1.00000 |
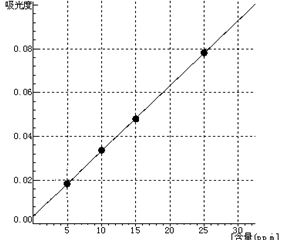 Ba Curve equation:y=0.0030*x+0.0036 Linearity coefficient:0.99988 |