Determination of Zinc and Aluminium Content in Alloys
1 Sample solution preparation:
4 Standard solution preparation
5 Standard curve
0.5g sample (precision to 0.0001g) was weighed and place in a PTFE beaker, wet with small amount of water. 10mL hydrofluoric acid, 10mL nitric acid, 3mL perchloric acid were added, covered, heated until the reaction is complete, the lid was opened and heated again until the white smoke stops, removed for cooling. 5mL (1+1) hydrochloric acid was added, heated to dissolve the salts, cooled, and transferred to a 50mL plastic volumetric flask, shaken well and set aside for later use.
2 Experimental equipment and reagents:
AA7000 series atomic absorption spectrophotometer (with Zn, Al hollow cathode lamp, EWAI Inc.)
Temperature-controlled hot plate
Nitric acid (HNO3): excellent grade purity
Hydrochloric acid (HCl): excellent grade purity
Hydrofluoric acid (HF): excellent grade purity
Perchloric acid (HClO4): excellent grade purity
Zinc standard solution (National Reference Materials Research Center)
Aluminium standard solution (National Reference Materials Research Center)
3 Instrument conditionsTemperature-controlled hot plate
Nitric acid (HNO3): excellent grade purity
Hydrochloric acid (HCl): excellent grade purity
Hydrofluoric acid (HF): excellent grade purity
Perchloric acid (HClO4): excellent grade purity
Zinc standard solution (National Reference Materials Research Center)
Aluminium standard solution (National Reference Materials Research Center)
| Parameter | Wavelength (nm) |
Slit width(nm) | Burner height(mm) | Fuel gas flow rate(L/min) | Lamp current(mA) | Flame type |
| Zn | 213.9 | 0.4 | 10 | 1.5 | 5.0 | Air – acetylene |
| Al | 309.3 | 0.2 | 8 | 3.0 | 3.0 | Air – acetylene |
4 Standard solution preparation
| Element | Concentration(μg/mL) | ||||
| Zn | 0 | 0.1 | 0.3 | 0.5 | 1.0 |
| Al | 0 | 1.0 | 5.0 | 10.0 | 25.0 |
5 Standard curve
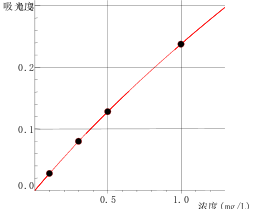 Zn Curve equation: Zn Curve equation:y=-0.03712x^2+0.27431x+0.00058 Linearity coefficient:1.00000 |
 Al Curve equation:y=0.0031*x+0.0038 Linearity coefficient:0.99981 |