Determination of K, Na, Ca and Mg Content in Washing Waste Water
1 Sample solution preparation
When detecting K and Na elements, it is sufficient to dilute sample directly to a relevant ratio with water. When detecting Ca and Mg elements, lanthanum salt was added to suppress ionization.
2 Experimental equipment and reagents
AA7020 series atomic absorption spectrophotometer (with Ca, Mg, K, Na hollow cathode lamp, EWAI Inc.)
Hydrochloric acid (HCl): excellent grade purity
100 g/L lanthanum chloride solution: 11.73g cerium oxide was weighed and placed in a 100mL volumetric flask, first wet with small amount of water, and then 37.5mL hydrochloric acid was added. Deionized water was added to dilute and make up the volume.
K standard solution (National Reference Materials Research Center)
Na standard solution (National Reference Materials Research Center)
Ca standard solution (National Reference Materials Research Center)
Mg standard solution (National Reference Materials Research Center)
3 Instrument conditions
4 Standard solution preparation
For every 100mL Mg and Ca standard solution, 1.5 mL 100 g/L lanthanum chloride solution should be added.
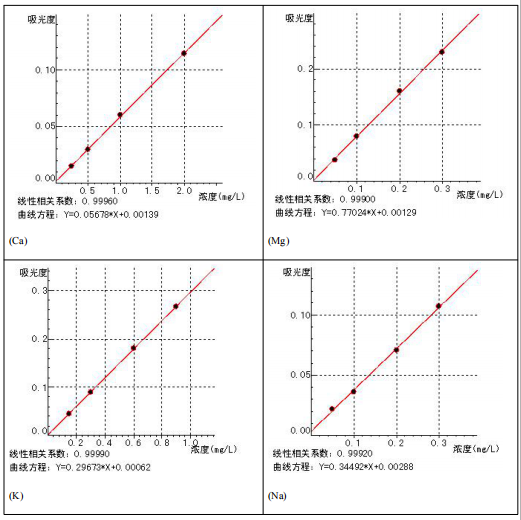
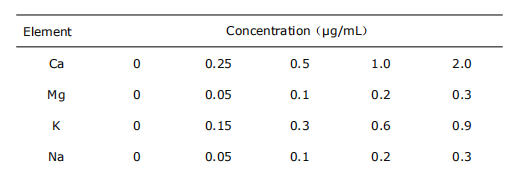 5 Standard curve
5 Standard curve
When detecting K and Na elements, it is sufficient to dilute sample directly to a relevant ratio with water. When detecting Ca and Mg elements, lanthanum salt was added to suppress ionization.
2 Experimental equipment and reagents
AA7020 series atomic absorption spectrophotometer (with Ca, Mg, K, Na hollow cathode lamp, EWAI Inc.)
Hydrochloric acid (HCl): excellent grade purity
100 g/L lanthanum chloride solution: 11.73g cerium oxide was weighed and placed in a 100mL volumetric flask, first wet with small amount of water, and then 37.5mL hydrochloric acid was added. Deionized water was added to dilute and make up the volume.
K standard solution (National Reference Materials Research Center)
Na standard solution (National Reference Materials Research Center)
Ca standard solution (National Reference Materials Research Center)
Mg standard solution (National Reference Materials Research Center)
3 Instrument conditions
| Parameter | Wavelength (nm) |
Slit width (nm) |
Burner height(mm) | Fuel gas flow rate(L/min) | Lamp current(mA) | Flame type |
| Ca | 422.7 | 0.2 | 8 | 1.5 | 3 | Air – acetylene |
| Mg | 285.2 | 0.2 | 8 | 1.5 | 2 | Air – acetylene |
| K | 766.5 | 0.2 | 8 | 1.3 | 3 | Air – acetylene |
| Na | 589.0 | 0.2 | 8 | 1.5 | 3 | Air - acetylene |
For every 100mL Mg and Ca standard solution, 1.5 mL 100 g/L lanthanum chloride solution should be added.