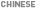Determination of Calcium, Magnesium, Nickel, Iron and Strontium Content in Brine
1 Sample solution preparation:
Chelating ion exchange resin (type 401) was taken and placed in a 10mm-ID exchange column up to about 10 cm high. After transformation with 1 mol/L HCl, it was washed with deionized water up to neutral, and then rinsed with 50 mL (pH = 12.0) water, ready for use. 100mL sample solution was taken and treated with concentrated alkali up to pH=12.0. It was then flowed through the treated resin at flow rate 1mL/min, then the resin was rinsed with 1mol/L HCl at flow rate 0.5mL/min, and 50mL eluent was collected which is to be tested.
2 Experimental equipment and reagents:
AA7000 series atomic absorption spectrophotometer (with Ca, Mg, Ni, Fe, Sr hollow cathode lamp, EWAI Inc.)
Exchange column with glass core
Hydrochloric acid (HCl): excellent grade purity
Sodium hydroxide (NaOH): excellent grade purity
100g / L lanthanum chloride solution: 11.73g of lanthanum oxide was weighed in a 100 mL volumetric flask, first wet with small amount of water and 37.5mL hydrochloric acid was added. Deionized water was added to dilute it up to the volume.
Calcium standard solution (National Reference Materials Research Center)
Magnesium standard solution (National Reference Materials Research Center)
Nickel standard solution (National Reference Materials Research Center)
Iron standard solution (National Reference Materials Research Center)
Strontium standard solution (National Reference Materials Research Center)
3 Instrument conditions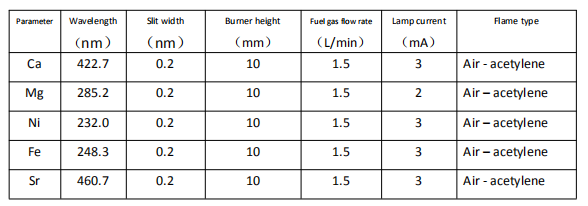 4 Standard solution preparation
4 Standard solution preparation
In every 100 mL Mg and Ca standard solution, 1.5 mL of 100 g/L lanthanum chloride solution is to be added respectively.
 5 Standard curve
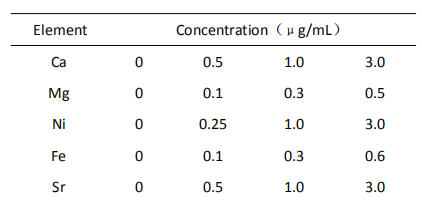
5 Standard curve
When measuring Ca and Mg sample solution, its lanthanum chloride concentration needs to be the same as that in standard solution.
Chelating ion exchange resin (type 401) was taken and placed in a 10mm-ID exchange column up to about 10 cm high. After transformation with 1 mol/L HCl, it was washed with deionized water up to neutral, and then rinsed with 50 mL (pH = 12.0) water, ready for use. 100mL sample solution was taken and treated with concentrated alkali up to pH=12.0. It was then flowed through the treated resin at flow rate 1mL/min, then the resin was rinsed with 1mol/L HCl at flow rate 0.5mL/min, and 50mL eluent was collected which is to be tested.
2 Experimental equipment and reagents:
AA7000 series atomic absorption spectrophotometer (with Ca, Mg, Ni, Fe, Sr hollow cathode lamp, EWAI Inc.)
Exchange column with glass core
Hydrochloric acid (HCl): excellent grade purity
Sodium hydroxide (NaOH): excellent grade purity
100g / L lanthanum chloride solution: 11.73g of lanthanum oxide was weighed in a 100 mL volumetric flask, first wet with small amount of water and 37.5mL hydrochloric acid was added. Deionized water was added to dilute it up to the volume.
Calcium standard solution (National Reference Materials Research Center)
Magnesium standard solution (National Reference Materials Research Center)
Nickel standard solution (National Reference Materials Research Center)
Iron standard solution (National Reference Materials Research Center)
Strontium standard solution (National Reference Materials Research Center)
3 Instrument conditions

In every 100 mL Mg and Ca standard solution, 1.5 mL of 100 g/L lanthanum chloride solution is to be added respectively.

When measuring Ca and Mg sample solution, its lanthanum chloride concentration needs to be the same as that in standard solution.